-
PDF
- Split View
-
Views
-
Cite
Cite
Alexandra Kovaleva, Hilde H. F. Remmelts, Ger T. Rijkers, Andy I. M. Hoepelman, Douwe H. Biesma, Jan Jelrik Oosterheert, Immunomodulatory effects of macrolides during community-acquired pneumonia: a literature review, Journal of Antimicrobial Chemotherapy, Volume 67, Issue 3, March 2012, Pages 530–540, https://doi.org/10.1093/jac/dkr520
Close - Share Icon Share
Abstract
Macrolides are known to possess immunomodulatory properties, next to their antimicrobial effects. These immunomodulatory activities have been proven beneficial in chronic pulmonary inflammatory diseases. Whether macrolides also exert favourable immunomodulatory effects during acute inflammation, and therefore can act as adjuvant therapy in community-acquired pneumonia (CAP), is less clear. We aimed to give an overview of the existing evidence from in vitro and in vivo studies on the immunomodulatory effects of macrolides during CAP. A comprehensive search in the PubMed/MEDLINE and Embase databases was performed. Two investigators independently examined the eligible literature. Studies that dealt with the effects of macrolides on the immune response, in terms of cytokine secretion and the number or function of inflammatory and structural cells during acute inflammation, were included. A total of 27 studies were included, of which 15 were in vitro studies, 9 in vivo, 2 both in vivo and in vitro, and 1 was in human subjects. Although the methods and experimental model systems used in these studies are very heterogeneous, macrolides in general tempered inflammation caused by viable and non-viable bacteria or their products. Cytokine secretion decreased, as did inflammatory and structural cell activation and histological inflammatory signs. Not all data, however, are consistent and sometimes pro-inflammatory effects were found. To conclude, the available literature suggests that macrolides can temper the inflammatory response during CAP, independent of their antimicrobial activity. However, because the studies differ in their methodology, no definite conclusions can be drawn.
Introduction
Despite effective antibiotic treatment and vaccination strategies, community-acquired pneumonia (CAP) still causes considerable morbidity and mortality. Lower respiratory tract infections are among the leading infectious causes of death in the developed world.1 Complications associated with CAP, such as severe lung injury, multiorgan failure and shock, result from a complex interplay of the effects of microorganisms and their products on the host, and the inflammatory reaction mediated by the host immune system. Although an adequate inflammatory response is necessary for the clearance of microorganisms, excessive inflammation can lead to ongoing local and systemic damage.2
In order to improve the outcome of CAP, research has focused on adjuvant therapy next to antibiotic treatment. These therapies are aimed at either the microorganism or the host, and target improvement of bacterial opsonization, improvement of effector mechanisms of the immune response and limitation of immunopathology. Promising treatment options in CAP include corticosteroids, macrolide antibiotics, statins, immunoglobulins, activated protein C, mannose-binding-lectin substitution therapy and Toll-like receptor antagonists.3
Macrolides are known to possess immunomodulatory properties beyond their direct antibacterial activities.4 The immunomodulatory effects of macrolides are beneficial in chronic pulmonary inflammatory syndromes, such as diffuse panbronchiolitis, cystic fibrosis, asthma and bronchiectasis. In these chronic diseases, the administration of macrolides is associated with a decrease in disease severity, length of hospital stay and mortality.5
Whether macrolides also exert favourable immunomodulatory effects during acute inflammatory conditions, such as CAP, is less clear. Worldwide, controversy still exists regarding the best empirical antimicrobial regimen for CAP. Several retrospective clinical studies have shown survival benefits in patients with CAP treated with macrolides in combination with β-lactam antibiotics, compared with patients treated with β-lactam monotherapy.6–11 However, due to the retrospective design of those studies, no definite conclusions can be drawn on whether the administration of macrolides to all patients treated for CAP is beneficial. More insight is needed into the exact mechanisms of the immunomodulatory actions of macrolides during acute inflammation. The aim of this review is to give an overview of the existing evidence from in vitro and in vivo studies on the immunomodulatory effects of macrolides during acute inflammation caused by microorganisms commonly responsible for CAP. We focus on the effects of macrolides on cytokine secretion, and on the number and function of inflammatory and structural cells of the respiratory tract.
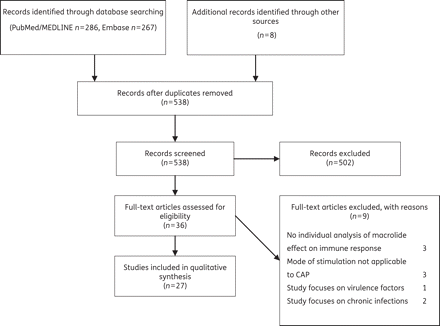
A comprehensive search was performed in the PubMed/MEDLINE and Embase databases. The search was limited to publications in the English language up to 22 August 2011. The following search terms were used: macrolides, azithromycin, erythromycin, clarithromycin, erythromycin estolate, erythromycin ethylsuccinate, ketolides, roxithromycin, cethromycin, telithromycin, immunomodulation, inflammation, inflammation mediators, cytokines, Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Mycoplasma, Chlamydia, Legionella and Coxiella. Two investigators independently screened the identified titles and abstracts. The full text of potentially relevant articles was examined. We searched the reference lists of retrieved studies for additional relevant reports. Studies that dealt with the effects of macrolides on the immune response were included. An effect on the immune response was defined as a change in cytokine secretion or a change in the number or function of inflammatory cells and structural cells of the respiratory tract. The search results and exclusion criteria are summarized in Figure 1.
The initial search yielded 286 references for PubMed/MEDLINE and 267 references for Embase. Eight additional studies were identified through a manual search. A total of 23 duplicates were removed, and 502 studies were excluded based on the title and abstract. Subsequently, 36 full-text articles were retrieved and screened. Eventually, we included and analysed 27 studies for this review (Figure 1).
Effects of macrolides on cytokine production
Cytokines are chemical messengers of the immune system that orchestrate the nature, intensity and duration of the immune response.12 The major pro-inflammatory cytokines are interleukin-1 (IL-1), tumour necrosis factor-α (TNF-α), IL-6 and IL-12. Chemokines are a subgroup of cytokines that affect chemotaxis. Examples include IL-8 (CXCL8), of which keratinocyte chemoattractant (KC) is the murine homologue, macrophage inflammatory protein-1 (MIP-1; CCL3), MIP-2 (CXCL2), monocyte chemoattractant protein-1 (MCP-1; CCL2), epithelial neutrophil-activating protein 78 (ENA-78; CXCL5), and regulated upon activation normal T cell expressed and secreted (RANTES; CCL5). Anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist, regulate the inflammatory response, e.g. by inhibiting pro-inflammatory cytokine production or by counteracting the effects of pro-inflammatory cytokines.13
In vitro cytokine studies
Several in vitro studies evaluated the effects of macrolides on the cytokine production of endothelial, epithelial and inflammatory cells exposed to pathogens commonly causing CAP. In general, these studies showed that macrolides have a suppressive effect on the cytokine secretion of several cell types (Table 1). This effect was found, irrespective of whether viable14,15 or non-viable bacteria,16–18 or bacterial products were used to stimulate the cells.19–24 This strengthens the notion that tempering of the immune response takes place during macrolide treatment. Particularly interesting is the comparison of macrolide treatment of the cells with treatment based on other antibiotics. For example, clarithromycin decreased IL-8 secretion by human alveolar type II cells when these cells were stimulated with crude antigens from Mycoplasma pneumoniae. This effect was absent when other antibiotics that are effective against M. pneumoniae (minocycline and ciprofloxacin) were used.20 A decrease in cytokine secretion from epithelial cells was evident in the case of infection with live bacteria after treatment with erythromycin, but not after gentamicin.15 Furthermore, cytokine secretion from whole blood and endothelium after stimulation with bacterial products was decreased during macrolide treatment in comparison with β-lactams.21,22 A similar effect was found when whole blood was stimulated with non-viable microorganisms.16 In contrast with the above-mentioned studies, in some publications macrolides did not influence cytokine secretion.25–28 This lack of an effect could be due to a true absence of immunomodulatory effects, but might also be due to the methods employed in the experiments, such as the mode of stimulation of the cell, the cell types used or the incubation times. Overall, the results from in vitro studies suggest that macrolides have a suppressive effect on cytokine secretion in models of acute inflammation. However, because of the heterogeneity of the studies and the obvious difficulties in extrapolating the results from in vitro to in vivo, these findings should be interpreted with caution.
Effects of macrolides on cytokine secretion in vitro sorted by cell type
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Nasal epithelium | human | ERY | S. pneumoniae | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | LPS and IL-1β | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | M. pneumoniae | — | IL-8, RANTES | there was no difference in cytokine secretion between infected and uninfected cells before ERY administration | Kazachkov et al. (2002)26 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | ↓ IL-8, TNF-α | — | 10 mg/L ERY reduced cytokines compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | ↓ IL-6, IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | ERY | IL-1β | ↓ IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | CLR | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | CLR | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Alveolar type II cells | human | CLR | M. pneumoniae antigen | ↓ IL-8 | — | CIP and MIN did not affect cytokine secretion | Kurata et al. (2010)20 |
| Alveolar cells | murine | TEL | LPS-stimulated macrophage supernatant | ↓ MIP-2 | — | — | Leiva et al. (2008)23 |
| Endothelium | human | ERY | S. aureus supernatant | ↓ IL-8, MCP-1 | — | compared with β-lactams | van Langevelde et al. (1999)22 |
| Endothelium | human | CLR | Chlamydia pneumoniae | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | ↓ IL-8a, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | ↓ IL-8, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Whole blood | human | ERY | S. aureus supernatant | ↓ IL-10, TNF-α | — | compared with β-lactams | van Langevelde et al. (1998)21 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | IL-10, IL-12 p40, IL-12 p70, IFN-γ | — | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, IL-10, IL-12 p40, IL-12 p70, TNF-α, IFN-γ | — | compared with penicillin | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-8, ENA-78 | — | — | Schultz et al. (2000)17 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | — | a decrease was evident with intravenous ERY and to a lesser extent with oral ERY | Guchelaar et al. (2001)18 |
| PMNs | human | ERY | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PMNs | human | AZM | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PBMCs | human | ERY | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | CLR | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | AZM | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | AZM inhibited more strongly than ERY and CLR | Kushiya et al. (2005)24 |
| Macrophages | murine | TEL | LPS | ↓ TNF-α, MIP-2 | — | TNF-α was also suppressed when LPS and TEL were added simultaneously | Leiva et al. (2008)23 |
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Nasal epithelium | human | ERY | S. pneumoniae | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | LPS and IL-1β | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | M. pneumoniae | — | IL-8, RANTES | there was no difference in cytokine secretion between infected and uninfected cells before ERY administration | Kazachkov et al. (2002)26 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | ↓ IL-8, TNF-α | — | 10 mg/L ERY reduced cytokines compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | ↓ IL-6, IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | ERY | IL-1β | ↓ IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | CLR | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | CLR | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Alveolar type II cells | human | CLR | M. pneumoniae antigen | ↓ IL-8 | — | CIP and MIN did not affect cytokine secretion | Kurata et al. (2010)20 |
| Alveolar cells | murine | TEL | LPS-stimulated macrophage supernatant | ↓ MIP-2 | — | — | Leiva et al. (2008)23 |
| Endothelium | human | ERY | S. aureus supernatant | ↓ IL-8, MCP-1 | — | compared with β-lactams | van Langevelde et al. (1999)22 |
| Endothelium | human | CLR | Chlamydia pneumoniae | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | ↓ IL-8a, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | ↓ IL-8, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Whole blood | human | ERY | S. aureus supernatant | ↓ IL-10, TNF-α | — | compared with β-lactams | van Langevelde et al. (1998)21 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | IL-10, IL-12 p40, IL-12 p70, IFN-γ | — | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, IL-10, IL-12 p40, IL-12 p70, TNF-α, IFN-γ | — | compared with penicillin | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-8, ENA-78 | — | — | Schultz et al. (2000)17 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | — | a decrease was evident with intravenous ERY and to a lesser extent with oral ERY | Guchelaar et al. (2001)18 |
| PMNs | human | ERY | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PMNs | human | AZM | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PBMCs | human | ERY | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | CLR | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | AZM | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | AZM inhibited more strongly than ERY and CLR | Kushiya et al. (2005)24 |
| Macrophages | murine | TEL | LPS | ↓ TNF-α, MIP-2 | — | TNF-α was also suppressed when LPS and TEL were added simultaneously | Leiva et al. (2008)23 |
↓, a significant decrease; AZM, azithromycin; CLR, clarithromycin; CIP, ciprofloxacin; ENA-78, epithelial neutrophil-activating protein 78; ERY, erythromycin; IFN, interferon; IL, interleukin; LPS, lipopolysaccharides; MCP, monocyte chemoattractant protein; MIN, minocycline; MIP, macrophage inflammatory protein; MXF, moxifloxacin; PBMCs, peripheral blood mononuclear cells; PMNs, polymorphonuclear leucocytes; RANTES, regulated upon activation normal T cell expressed and secreted; RXM, roxithromycin; TEL, telithromycin; TNF, tumour necrosis factor; TSST, toxic shock syndrome toxin.
aA downward trend was perceived, but the decrease never reached significance.
Effects of macrolides on cytokine secretion in vitro sorted by cell type
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Nasal epithelium | human | ERY | S. pneumoniae | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | LPS and IL-1β | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | M. pneumoniae | — | IL-8, RANTES | there was no difference in cytokine secretion between infected and uninfected cells before ERY administration | Kazachkov et al. (2002)26 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | ↓ IL-8, TNF-α | — | 10 mg/L ERY reduced cytokines compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | ↓ IL-6, IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | ERY | IL-1β | ↓ IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | CLR | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | CLR | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Alveolar type II cells | human | CLR | M. pneumoniae antigen | ↓ IL-8 | — | CIP and MIN did not affect cytokine secretion | Kurata et al. (2010)20 |
| Alveolar cells | murine | TEL | LPS-stimulated macrophage supernatant | ↓ MIP-2 | — | — | Leiva et al. (2008)23 |
| Endothelium | human | ERY | S. aureus supernatant | ↓ IL-8, MCP-1 | — | compared with β-lactams | van Langevelde et al. (1999)22 |
| Endothelium | human | CLR | Chlamydia pneumoniae | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | ↓ IL-8a, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | ↓ IL-8, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Whole blood | human | ERY | S. aureus supernatant | ↓ IL-10, TNF-α | — | compared with β-lactams | van Langevelde et al. (1998)21 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | IL-10, IL-12 p40, IL-12 p70, IFN-γ | — | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, IL-10, IL-12 p40, IL-12 p70, TNF-α, IFN-γ | — | compared with penicillin | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-8, ENA-78 | — | — | Schultz et al. (2000)17 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | — | a decrease was evident with intravenous ERY and to a lesser extent with oral ERY | Guchelaar et al. (2001)18 |
| PMNs | human | ERY | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PMNs | human | AZM | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PBMCs | human | ERY | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | CLR | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | AZM | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | AZM inhibited more strongly than ERY and CLR | Kushiya et al. (2005)24 |
| Macrophages | murine | TEL | LPS | ↓ TNF-α, MIP-2 | — | TNF-α was also suppressed when LPS and TEL were added simultaneously | Leiva et al. (2008)23 |
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|---|
| Nasal epithelium | human | ERY | S. pneumoniae | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | LPS and IL-1β | — | IL-8 | — | Lagrou et al. (2000)25 |
| Nasal epithelium | human | ERY | M. pneumoniae | — | IL-8, RANTES | there was no difference in cytokine secretion between infected and uninfected cells before ERY administration | Kazachkov et al. (2002)26 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | ↓ IL-8, TNF-α | — | 10 mg/L ERY reduced cytokines compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | ↓ IL-6, IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | ERY | IL-1β | ↓ IL-8 | — | — | Khair et al. (1995)19 |
| Bronchial epithelium | human | CLR | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | CLR | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | M. pneumoniae membrane fraction | — | IL-8 | MXF did not inhibit IL-8 either | Chmura et al. (2008)28 |
| Bronchial epithelium | human | AZM | TNF-α | ↓ IL-8 | — | MXF inhibited IL-8, but only at the highest concentrations | Chmura et al. (2008)28 |
| Alveolar type II cells | human | CLR | M. pneumoniae antigen | ↓ IL-8 | — | CIP and MIN did not affect cytokine secretion | Kurata et al. (2010)20 |
| Alveolar cells | murine | TEL | LPS-stimulated macrophage supernatant | ↓ MIP-2 | — | — | Leiva et al. (2008)23 |
| Endothelium | human | ERY | S. aureus supernatant | ↓ IL-8, MCP-1 | — | compared with β-lactams | van Langevelde et al. (1999)22 |
| Endothelium | human | CLR | Chlamydia pneumoniae | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | — | IL-8, MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | ↓ IL-8a, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | ↓ IL-8, MCP-1 | — | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | ↓ IL-8 | MCP-1 | — | Uriarte et al. (2002)14 |
| Whole blood | human | ERY | S. aureus supernatant | ↓ IL-10, TNF-α | — | compared with β-lactams | van Langevelde et al. (1998)21 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | IL-10, IL-12 p40, IL-12 p70, IFN-γ | — | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, IL-10, IL-12 p40, IL-12 p70, TNF-α, IFN-γ | — | compared with penicillin | Schultz et al. (1998)16 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-8, ENA-78 | — | — | Schultz et al. (2000)17 |
| Whole blood | human | ERY | S. pneumoniae, killed | ↓ IL-6, TNF-α | — | a decrease was evident with intravenous ERY and to a lesser extent with oral ERY | Guchelaar et al. (2001)18 |
| PMNs | human | ERY | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PMNs | human | AZM | S. pneumoniae lysate | — | IL-8 | short incubation time | Koch et al. (2000)27 |
| PBMCs | human | ERY | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | CLR | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | Kushiya et al. (2005)24 | |
| PBMCs | human | AZM | TSST-1 | ↓ IL-2, TNF-α, IFN-γ | — | AZM inhibited more strongly than ERY and CLR | Kushiya et al. (2005)24 |
| Macrophages | murine | TEL | LPS | ↓ TNF-α, MIP-2 | — | TNF-α was also suppressed when LPS and TEL were added simultaneously | Leiva et al. (2008)23 |
↓, a significant decrease; AZM, azithromycin; CLR, clarithromycin; CIP, ciprofloxacin; ENA-78, epithelial neutrophil-activating protein 78; ERY, erythromycin; IFN, interferon; IL, interleukin; LPS, lipopolysaccharides; MCP, monocyte chemoattractant protein; MIN, minocycline; MIP, macrophage inflammatory protein; MXF, moxifloxacin; PBMCs, peripheral blood mononuclear cells; PMNs, polymorphonuclear leucocytes; RANTES, regulated upon activation normal T cell expressed and secreted; RXM, roxithromycin; TEL, telithromycin; TNF, tumour necrosis factor; TSST, toxic shock syndrome toxin.
aA downward trend was perceived, but the decrease never reached significance.
In vivo cytokine studies
Next to in vitro experiments, several in vivo studies have investigated the effects of macrolides on cytokine secretion during acute inflammation. The findings of these studies are listed in detail in Table 2.
Effects of macrolides on cytokine secretion in vivo sorted by pathogen/stimulation
| Pathogen/stimulation . | Model . | Macrolide . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| S. pneumoniae | murine | HMR 3004 | ↓ IL-6 | — | compared with placebo | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | murine | RXM | ↓ KC, ↑ MCP-1 | IL-1β, TNF-α | compared with placebo | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | murine | HMR 3004 | ↓ IL-1β, IL-6 | TNF-α | compared with placebo | Duong et al. (1998)37 |
| M. pneumoniae | murine | CLR | ↓ IL-6, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-10 | no significant difference in BAL bacterial cultures between placebo and CLR-treated animals | Hardy et al. (2003)31 |
| M. pneumoniae | murine | CLR | ↓ IL-12 p40, KC, MCP-1, RANTES | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12 p70, IL-13, IL-17, TNF-α, IFN-γ, MIP-1α, MIP-1β, G-CSF, GM-CSF, PDGF, VEGF, eotaxin | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | murine | AZM | ↓ IL-12, TNF-α, KC, MCP-1, MIP-1α | IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, GM-CSF | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | murine | CET | ↓ IL-1β, IL-12, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-2, IL-4, GM-CSF | cytokines decreased, whereas BAL cultures did not differ between the CET group and the placebo group | Rios et al. (2004)30 |
| M. pneumoniae, killed | murine | CLR | ↑ IL-6 | IL-10, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | compared with placebo | Hardy et al. (2003)31 |
| Mycoplasma extract | murine | CLR | ↑ IL-6, TNF-α, MCP-1, MIP-1α RANTES | IL-17, IL-23, KC | no increase in total cell and PMN counts in the lung despite higher cytokine concentrations in BALF | Hirao et al. (2011)36 |
| H. influenzae, macrolide resistant | murine | CLR | ↓ IL-1β, MIP-2 | — | macrolide treatment reduced bacterial cultures despite resistance in high-dose treatment group; cytokine levels were reduced in low-dose treatment group despite no significant reduction in bacterial cultures | Nakamura et al. (2010)34 |
| LPS | murine | TEL | ↓ TNF-α, MIP-2 | — | compared with placebo | Leiva et al. (2008)23 |
| Unknown | human | CLR | ↓ IL-6, ↑ IL-10, IFN-γ | — | compared with amoxicillin | Demartini et al. (2004)39 |
| Pathogen/stimulation . | Model . | Macrolide . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| S. pneumoniae | murine | HMR 3004 | ↓ IL-6 | — | compared with placebo | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | murine | RXM | ↓ KC, ↑ MCP-1 | IL-1β, TNF-α | compared with placebo | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | murine | HMR 3004 | ↓ IL-1β, IL-6 | TNF-α | compared with placebo | Duong et al. (1998)37 |
| M. pneumoniae | murine | CLR | ↓ IL-6, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-10 | no significant difference in BAL bacterial cultures between placebo and CLR-treated animals | Hardy et al. (2003)31 |
| M. pneumoniae | murine | CLR | ↓ IL-12 p40, KC, MCP-1, RANTES | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12 p70, IL-13, IL-17, TNF-α, IFN-γ, MIP-1α, MIP-1β, G-CSF, GM-CSF, PDGF, VEGF, eotaxin | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | murine | AZM | ↓ IL-12, TNF-α, KC, MCP-1, MIP-1α | IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, GM-CSF | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | murine | CET | ↓ IL-1β, IL-12, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-2, IL-4, GM-CSF | cytokines decreased, whereas BAL cultures did not differ between the CET group and the placebo group | Rios et al. (2004)30 |
| M. pneumoniae, killed | murine | CLR | ↑ IL-6 | IL-10, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | compared with placebo | Hardy et al. (2003)31 |
| Mycoplasma extract | murine | CLR | ↑ IL-6, TNF-α, MCP-1, MIP-1α RANTES | IL-17, IL-23, KC | no increase in total cell and PMN counts in the lung despite higher cytokine concentrations in BALF | Hirao et al. (2011)36 |
| H. influenzae, macrolide resistant | murine | CLR | ↓ IL-1β, MIP-2 | — | macrolide treatment reduced bacterial cultures despite resistance in high-dose treatment group; cytokine levels were reduced in low-dose treatment group despite no significant reduction in bacterial cultures | Nakamura et al. (2010)34 |
| LPS | murine | TEL | ↓ TNF-α, MIP-2 | — | compared with placebo | Leiva et al. (2008)23 |
| Unknown | human | CLR | ↓ IL-6, ↑ IL-10, IFN-γ | — | compared with amoxicillin | Demartini et al. (2004)39 |
↓, a significant decrease; ↑, a significant increase; AZM, azithromycin; BAL, bronchoalveolar lavage; BALF, bronchoalveolar lavage fluid; CLR, clarithromycin; CET, cethromycin; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukin; IFN, interferon; KC, keratinocyte chemoattractant; LPS, lipopolysaccharides; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; PDGF, platelet-derived growth factor; PMN, polymorphonuclear leucocyte; RANTES, regulated upon activation normal T cell expressed and secreted; RXM, roxithromycin; TEL, telithromycin; TNF, tumour necrosis factor; VEGF, vascular endothelium growth factor.
Effects of macrolides on cytokine secretion in vivo sorted by pathogen/stimulation
| Pathogen/stimulation . | Model . | Macrolide . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| S. pneumoniae | murine | HMR 3004 | ↓ IL-6 | — | compared with placebo | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | murine | RXM | ↓ KC, ↑ MCP-1 | IL-1β, TNF-α | compared with placebo | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | murine | HMR 3004 | ↓ IL-1β, IL-6 | TNF-α | compared with placebo | Duong et al. (1998)37 |
| M. pneumoniae | murine | CLR | ↓ IL-6, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-10 | no significant difference in BAL bacterial cultures between placebo and CLR-treated animals | Hardy et al. (2003)31 |
| M. pneumoniae | murine | CLR | ↓ IL-12 p40, KC, MCP-1, RANTES | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12 p70, IL-13, IL-17, TNF-α, IFN-γ, MIP-1α, MIP-1β, G-CSF, GM-CSF, PDGF, VEGF, eotaxin | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | murine | AZM | ↓ IL-12, TNF-α, KC, MCP-1, MIP-1α | IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, GM-CSF | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | murine | CET | ↓ IL-1β, IL-12, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-2, IL-4, GM-CSF | cytokines decreased, whereas BAL cultures did not differ between the CET group and the placebo group | Rios et al. (2004)30 |
| M. pneumoniae, killed | murine | CLR | ↑ IL-6 | IL-10, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | compared with placebo | Hardy et al. (2003)31 |
| Mycoplasma extract | murine | CLR | ↑ IL-6, TNF-α, MCP-1, MIP-1α RANTES | IL-17, IL-23, KC | no increase in total cell and PMN counts in the lung despite higher cytokine concentrations in BALF | Hirao et al. (2011)36 |
| H. influenzae, macrolide resistant | murine | CLR | ↓ IL-1β, MIP-2 | — | macrolide treatment reduced bacterial cultures despite resistance in high-dose treatment group; cytokine levels were reduced in low-dose treatment group despite no significant reduction in bacterial cultures | Nakamura et al. (2010)34 |
| LPS | murine | TEL | ↓ TNF-α, MIP-2 | — | compared with placebo | Leiva et al. (2008)23 |
| Unknown | human | CLR | ↓ IL-6, ↑ IL-10, IFN-γ | — | compared with amoxicillin | Demartini et al. (2004)39 |
| Pathogen/stimulation . | Model . | Macrolide . | Influence on cytokine secretion . | No influence on cytokine secretion . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| S. pneumoniae | murine | HMR 3004 | ↓ IL-6 | — | compared with placebo | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | murine | RXM | ↓ KC, ↑ MCP-1 | IL-1β, TNF-α | compared with placebo | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | murine | HMR 3004 | ↓ IL-1β, IL-6 | TNF-α | compared with placebo | Duong et al. (1998)37 |
| M. pneumoniae | murine | CLR | ↓ IL-6, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-10 | no significant difference in BAL bacterial cultures between placebo and CLR-treated animals | Hardy et al. (2003)31 |
| M. pneumoniae | murine | CLR | ↓ IL-12 p40, KC, MCP-1, RANTES | IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12 p70, IL-13, IL-17, TNF-α, IFN-γ, MIP-1α, MIP-1β, G-CSF, GM-CSF, PDGF, VEGF, eotaxin | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | murine | AZM | ↓ IL-12, TNF-α, KC, MCP-1, MIP-1α | IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, GM-CSF | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | murine | CET | ↓ IL-1β, IL-12, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | IL-2, IL-4, GM-CSF | cytokines decreased, whereas BAL cultures did not differ between the CET group and the placebo group | Rios et al. (2004)30 |
| M. pneumoniae, killed | murine | CLR | ↑ IL-6 | IL-10, TNF-α, IFN-γ, KC, MCP-1, MIP-1α | compared with placebo | Hardy et al. (2003)31 |
| Mycoplasma extract | murine | CLR | ↑ IL-6, TNF-α, MCP-1, MIP-1α RANTES | IL-17, IL-23, KC | no increase in total cell and PMN counts in the lung despite higher cytokine concentrations in BALF | Hirao et al. (2011)36 |
| H. influenzae, macrolide resistant | murine | CLR | ↓ IL-1β, MIP-2 | — | macrolide treatment reduced bacterial cultures despite resistance in high-dose treatment group; cytokine levels were reduced in low-dose treatment group despite no significant reduction in bacterial cultures | Nakamura et al. (2010)34 |
| LPS | murine | TEL | ↓ TNF-α, MIP-2 | — | compared with placebo | Leiva et al. (2008)23 |
| Unknown | human | CLR | ↓ IL-6, ↑ IL-10, IFN-γ | — | compared with amoxicillin | Demartini et al. (2004)39 |
↓, a significant decrease; ↑, a significant increase; AZM, azithromycin; BAL, bronchoalveolar lavage; BALF, bronchoalveolar lavage fluid; CLR, clarithromycin; CET, cethromycin; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukin; IFN, interferon; KC, keratinocyte chemoattractant; LPS, lipopolysaccharides; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; PDGF, platelet-derived growth factor; PMN, polymorphonuclear leucocyte; RANTES, regulated upon activation normal T cell expressed and secreted; RXM, roxithromycin; TEL, telithromycin; TNF, tumour necrosis factor; VEGF, vascular endothelium growth factor.
Ten studies examined the effects of macrolides on cytokine production during acute inflammation in murine pneumonia models. In five studies, macrolides decreased the concentration of cytokines and chemokines in bronchoalveolar lavage fluid (BALF) of mice infected with live bacteria.29–33 In one of these experiments, cytokine secretion was decreased significantly while no effect on bacterial counts was found.30 Two murine studies also found a decrease in the secretion of cytokines when the infection was caused by macrolide-resistant microorganisms.34,35 These data suggest that macrolides have immunomodulatory activities independent of their direct antibacterial effects. When acute inflammation was induced by killed microorganisms or bacterial products, however, conflicting results were found. Clarithromycin treatment of pneumonia induced with UV-killed M. pneumoniae caused no decline in cytokine secretion.31 Surprisingly, BALF cytokine concentrations were even higher in clarithromycin-treated animals. Similarly, no decline, but rather a rise in cytokine secretion was found when infection with Mycoplasma extract was treated with clarithromycin.36 In contrast, the ketolide HMR 3004 did decrease cytokine secretion in animals inoculated with non-viable S. pneumoniae.37 Another study evaluated a model in which pulmonary inflammation was induced by lipopolysaccharides (LPS). Here, treatment with telithromycin resulted in an attenuation of the cytokine response compared with untreated controls.23 This illustrates that studies yielded very heterogeneous results when different modes of stimulation and macrolides were used, especially in the case of non-viable bacteria or bacterial products.
Although the studies in murine pneumonia models are very heterogeneous with regard to methodology and some of the results, there were several important consistent observations. In general, macrolides decreased cytokine levels in murine models of pneumonia induced with viable microorganisms. However, it is difficult to conclude whether this modulation of cytokine secretion is due to direct antimicrobial activity or to immunomodulatory effects of the macrolide in question. When macrolide-resistant microorganisms were used to infect the animals, a decrease in cytokine levels was still found. This suggests an immunomodulatory effect, independent of a direct antibacterial effect. However, macrolides are claimed to accumulate in phagocytic cells and epithelial lining fluid,38 possibly causing drug concentrations to rise well above the MIC. Nevertheless, in one study, the cytokine response already decreased while bacterial loads had not declined significantly, as would have been expected if the antimicrobial activity of macrolides was primarily responsible for the attenuation of the inflammatory response. This favours the concept of an immunomodulatory effect of macrolides. When pulmonary inflammation was induced with non-viable microorganisms, in some studies macrolides did not decrease cytokine secretion. This may mean that antimicrobial properties of macrolides predominate in the reduction of cytokine secretion. However, a decrease in cytokines was still found when acute lung inflammation was induced by LPS. A decrease would not be expected if only antimicrobial effects were exerted by macrolides. These findings together lead to the conclusion that the decline in cytokine levels found in murine models of pneumonia cannot be solely attributed to direct antimicrobial effects of macrolides, but must also be due to immunomodulatory activities.
One single in vivo study described the effect of macrolides on cytokine levels in human subjects with CAP. In this study, the effect of clarithromycin treatment was compared with that of amoxicillin. Treatment with clarithromycin resulted in a decrease in IL-6, and an increase in interferon-γ (IFN-γ) and IL-10 on the third and seventh day. In the patients treated with amoxicillin, no effect was found on IL-10, IFN-γ secretion decreased and a decline in IL-6 was evident only after 7 days.39 The proposed explanation by the authors is that this decrease was probably related to the resolution of inflammatory symptoms. This study suggests that clarithromycin can modulate cytokine production in vivo in humans with CAP. However, due to the small sample size of the study and uncertainty about the methods employed, no definite conclusions can be drawn.
Although it is evident that macrolides decrease cytokine secretion, it has to be kept in mind that extrapolation of in vitro as well as animal studies to clinical practice should be done with caution, because to date there is no measure of inflammation by cytokines that has been calibrated. Well-designed clinical studies are required to correlate the degree of clinical inflammation with a comprehensive analysis of cytokine patterns at the relevant body site.
Effects of macrolides on inflammatory cells
Polymorphonuclear leucocytes (PMNs) are the predominant cells that infiltrate tissue in the early stages of an inflammatory response. During this time, chemokines activate PMNs, which results in the adhesion of these inflammatory cells to e.g. endothelium, and subsequent transendothelial and transepithelial migration.12 Activating signals stimulate metabolic pathways to generate a respiratory burst in PMNs, which produce reactive oxygen species and reactive nitrogen species. Although these substances play an important role in the killing of various microorganisms, they can also contribute to tissue damage in the case of an excessive inflammatory response.40
In vitro studies on inflammatory cells
The effects of macrolides on inflammatory cells have been investigated in a number of in vitro studies, of which the results are depicted in Table 3. One study showed that PMNs that had been treated with azithromycin became committed to programmed cell death.27 Incubation with erythromycin, penicillin or dexamethasone did not produce a similar increase in apoptosis. The addition of bacterial lysate of S. pneumoniae to PMNs inhibited the induction of apoptosis by azithromycin treatment.27 Murine macrophages that were pre-treated with telithromycin and subsequently challenged with LPS also showed increased apoptosis.23 Azithromycin did not affect the oxidative function of PMNs when these cells were challenged with S. pneumoniae.27 Both telithromycin and roxithromycin, however, completely arrested the oxidative metabolism in PMNs, whilst not affecting the bactericidal ability of these cells when stimulated with S. aureus.41 In whole blood from healthy individuals treated with erythromycin in vivo and stimulated with heat-killed S. pneumoniae ex vivo, enhanced degranulation of both azurophilic and specific granules in PMNs was found.17 The combination of azithromycin with PMNs has been shown to augment the opsonophagocytic killing of S. aureus at concentrations above and below the MIC.42
Effects of macrolides on inflammatory cells sorted by pathogen/stimulation
| Pathogen/stimulation . | Model . | Macrolide . | Influence on inflammatory cells . | Comments . | Reference . |
|---|---|---|---|---|---|
| None | in vitro | ERY | ↑ apoptosis of PMNs without addition of S. pneumoniae lysate | addition of S. pneumoniae lysate abrogated apoptosis; PEN, ERY or DEXA did not induce apoptosis | Koch et al. (2000)27 |
| S. pneumoniae | in vitro | AZM | no effect on oxidative burst PMNs | short incubation time | Koch et al. (2000)27 |
| S. pneumoniae | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ monocytes, total cell counts and NO in BALF | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | in vivo | RXM | ↓ MPO activity (PMNs) in lung tissue | compared with untreated controls; mononuclear phagocytes predominated in treated animals | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vitro | ERY | ↑ degranulation of specific and azurophilic granules in PMNs | compared with situation before infusion of antibiotics | Schultz et al. (2000)17 |
| S. pneumoniae, killed | in vivo | RXM | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ total cell counts and NO in BALF | compared with untreated controls | Duong et al. (1998)37 |
| H. influenzae, macrolide resistant | in vivo | CLR | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Nakamura et al. (2010)34 |
| H. influenzae, endotoxin | in vitro | ERY | ↓ chemoattraction of PMNs to bronchial epithelium | compared with untreated controls | Khair et al. (1995)19 |
| Mycoplasma extract | in vivo | CLR | ↓ total cell counts and PMN counts BALF | a decrease in cells was evident despite an increase in cytokine secretion; compared with vehicle-treated controls | Hirao et al. (2011)36 |
| S. aureus | in vitro | AZM | ↑ opsonophagocytic quality of PMNs | compared with RP7293, diacetyl midecamycin and ERY | Herrera-Insua et al. (1997)42 |
| S. aureus | in vitro | RXM | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| S. aureus | in vitro | TEL | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| LPS | in vivo | TEL | ↓ total cell counts, PMN counts and NO in BALF | compared with untreated controls | Leiva et al. (2008)23 |
| LPS | in vitro | TEL | ↑ apoptosis of murine macrophages | compared with untreated controls | Leiva et al. (2008)23 |
| Pathogen/stimulation . | Model . | Macrolide . | Influence on inflammatory cells . | Comments . | Reference . |
|---|---|---|---|---|---|
| None | in vitro | ERY | ↑ apoptosis of PMNs without addition of S. pneumoniae lysate | addition of S. pneumoniae lysate abrogated apoptosis; PEN, ERY or DEXA did not induce apoptosis | Koch et al. (2000)27 |
| S. pneumoniae | in vitro | AZM | no effect on oxidative burst PMNs | short incubation time | Koch et al. (2000)27 |
| S. pneumoniae | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ monocytes, total cell counts and NO in BALF | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | in vivo | RXM | ↓ MPO activity (PMNs) in lung tissue | compared with untreated controls; mononuclear phagocytes predominated in treated animals | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vitro | ERY | ↑ degranulation of specific and azurophilic granules in PMNs | compared with situation before infusion of antibiotics | Schultz et al. (2000)17 |
| S. pneumoniae, killed | in vivo | RXM | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ total cell counts and NO in BALF | compared with untreated controls | Duong et al. (1998)37 |
| H. influenzae, macrolide resistant | in vivo | CLR | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Nakamura et al. (2010)34 |
| H. influenzae, endotoxin | in vitro | ERY | ↓ chemoattraction of PMNs to bronchial epithelium | compared with untreated controls | Khair et al. (1995)19 |
| Mycoplasma extract | in vivo | CLR | ↓ total cell counts and PMN counts BALF | a decrease in cells was evident despite an increase in cytokine secretion; compared with vehicle-treated controls | Hirao et al. (2011)36 |
| S. aureus | in vitro | AZM | ↑ opsonophagocytic quality of PMNs | compared with RP7293, diacetyl midecamycin and ERY | Herrera-Insua et al. (1997)42 |
| S. aureus | in vitro | RXM | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| S. aureus | in vitro | TEL | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| LPS | in vivo | TEL | ↓ total cell counts, PMN counts and NO in BALF | compared with untreated controls | Leiva et al. (2008)23 |
| LPS | in vitro | TEL | ↑ apoptosis of murine macrophages | compared with untreated controls | Leiva et al. (2008)23 |
↓, a significant decrease; ↑, a significant increase; AZM, azithromycin; BALF, bronchoalveolar lavage fluid; CLR, clarithromycin; DEXA, dexamethasone; ERY, erythromycin; LPS, lipopolysaccharides; MPO, myeloperoxidase; NO, nitric oxide; PEN, penicillin; PMNs, polymorphonuclear leucocytes; RXM, roxithromycin; TEL, telithromycin.
Effects of macrolides on inflammatory cells sorted by pathogen/stimulation
| Pathogen/stimulation . | Model . | Macrolide . | Influence on inflammatory cells . | Comments . | Reference . |
|---|---|---|---|---|---|
| None | in vitro | ERY | ↑ apoptosis of PMNs without addition of S. pneumoniae lysate | addition of S. pneumoniae lysate abrogated apoptosis; PEN, ERY or DEXA did not induce apoptosis | Koch et al. (2000)27 |
| S. pneumoniae | in vitro | AZM | no effect on oxidative burst PMNs | short incubation time | Koch et al. (2000)27 |
| S. pneumoniae | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ monocytes, total cell counts and NO in BALF | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | in vivo | RXM | ↓ MPO activity (PMNs) in lung tissue | compared with untreated controls; mononuclear phagocytes predominated in treated animals | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vitro | ERY | ↑ degranulation of specific and azurophilic granules in PMNs | compared with situation before infusion of antibiotics | Schultz et al. (2000)17 |
| S. pneumoniae, killed | in vivo | RXM | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ total cell counts and NO in BALF | compared with untreated controls | Duong et al. (1998)37 |
| H. influenzae, macrolide resistant | in vivo | CLR | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Nakamura et al. (2010)34 |
| H. influenzae, endotoxin | in vitro | ERY | ↓ chemoattraction of PMNs to bronchial epithelium | compared with untreated controls | Khair et al. (1995)19 |
| Mycoplasma extract | in vivo | CLR | ↓ total cell counts and PMN counts BALF | a decrease in cells was evident despite an increase in cytokine secretion; compared with vehicle-treated controls | Hirao et al. (2011)36 |
| S. aureus | in vitro | AZM | ↑ opsonophagocytic quality of PMNs | compared with RP7293, diacetyl midecamycin and ERY | Herrera-Insua et al. (1997)42 |
| S. aureus | in vitro | RXM | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| S. aureus | in vitro | TEL | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| LPS | in vivo | TEL | ↓ total cell counts, PMN counts and NO in BALF | compared with untreated controls | Leiva et al. (2008)23 |
| LPS | in vitro | TEL | ↑ apoptosis of murine macrophages | compared with untreated controls | Leiva et al. (2008)23 |
| Pathogen/stimulation . | Model . | Macrolide . | Influence on inflammatory cells . | Comments . | Reference . |
|---|---|---|---|---|---|
| None | in vitro | ERY | ↑ apoptosis of PMNs without addition of S. pneumoniae lysate | addition of S. pneumoniae lysate abrogated apoptosis; PEN, ERY or DEXA did not induce apoptosis | Koch et al. (2000)27 |
| S. pneumoniae | in vitro | AZM | no effect on oxidative burst PMNs | short incubation time | Koch et al. (2000)27 |
| S. pneumoniae | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ monocytes, total cell counts and NO in BALF | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | in vivo | RXM | ↓ MPO activity (PMNs) in lung tissue | compared with untreated controls; mononuclear phagocytes predominated in treated animals | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vitro | ERY | ↑ degranulation of specific and azurophilic granules in PMNs | compared with situation before infusion of antibiotics | Schultz et al. (2000)17 |
| S. pneumoniae, killed | in vivo | RXM | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | in vivo | HMR 3004 | ↓ MPO activity (PMNs) in lung tissue; ↓ total cell counts and NO in BALF | compared with untreated controls | Duong et al. (1998)37 |
| H. influenzae, macrolide resistant | in vivo | CLR | ↓ total cell counts and PMN counts in BALF | compared with untreated controls | Nakamura et al. (2010)34 |
| H. influenzae, endotoxin | in vitro | ERY | ↓ chemoattraction of PMNs to bronchial epithelium | compared with untreated controls | Khair et al. (1995)19 |
| Mycoplasma extract | in vivo | CLR | ↓ total cell counts and PMN counts BALF | a decrease in cells was evident despite an increase in cytokine secretion; compared with vehicle-treated controls | Hirao et al. (2011)36 |
| S. aureus | in vitro | AZM | ↑ opsonophagocytic quality of PMNs | compared with RP7293, diacetyl midecamycin and ERY | Herrera-Insua et al. (1997)42 |
| S. aureus | in vitro | RXM | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| S. aureus | in vitro | TEL | ↓ oxidative burst | bactericidal activity was unaffected despite attenuation of the oxidative burst | Vazifeh et al. (2002)41 |
| LPS | in vivo | TEL | ↓ total cell counts, PMN counts and NO in BALF | compared with untreated controls | Leiva et al. (2008)23 |
| LPS | in vitro | TEL | ↑ apoptosis of murine macrophages | compared with untreated controls | Leiva et al. (2008)23 |
↓, a significant decrease; ↑, a significant increase; AZM, azithromycin; BALF, bronchoalveolar lavage fluid; CLR, clarithromycin; DEXA, dexamethasone; ERY, erythromycin; LPS, lipopolysaccharides; MPO, myeloperoxidase; NO, nitric oxide; PEN, penicillin; PMNs, polymorphonuclear leucocytes; RXM, roxithromycin; TEL, telithromycin.
In vivo studies on inflammatory cells
Various murine pneumonia studies described the effects of macrolides on inflammatory cells in vivo (Table 3). Clarithromycin reduced both total cell counts and PMN counts in BALF in a murine model of H. influenzae pneumonia and pneumonia induced with Mycoplasma extract.34,36 In pneumonia caused by macrolide-resistant S. pneumoniae, a reduction in PMN cell infiltration in lungs was found in mice treated with roxithromycin, but compared with the control group the lungs of the treated animals showed a greater influx of mononuclear phagocytes. Despite the reduction in PMNs, bacterial counts were lower in treated mice. This was attributed to the increase in mononuclear cells. Moreover, when mice were inoculated with killed microorganisms, the inflammatory response expressed by total cell counts in BALF was also reduced in treated animals.35 HMR 3004 treatment of UV-killed S. pneumoniae pneumonia in mice resulted in lower PMN counts in BALF, compared with untreated mice. Moreover, there was an evident decline in nitric oxide (NO) release in the treatment group when compared with untreated animals.37 HMR 3004 administration to viable S. pneumoniae pneumonia also resulted in a reduction of NO to levels comparable to those found in uninfected animals.32 Telithromycin even decreased NO production in mice infected with LPS.23 Moreover, lower total cell counts and PMN counts in BALF were found, compared with untreated mice.
From these studies, it can be concluded that the behaviour of inflammatory cells changes to a more anti-inflammatory nature as a result of macrolide treatment. Inflammatory cells in murine pneumonia models that were treated with macrolides accumulated to a lesser degree in the affected lung compartments. This effect has been demonstrated in pneumonia caused by viable and non-viable bacteria or their products. These effects are promising for clinical practice, because reduced accumulation of PMNs may result in less collateral damage to the lung. Another possible benefit is the finding of attenuation of NO release by PMNs after macrolide treatment, because excessive NO release can result in damage to lung tissue. Murine macrophages and human PMNs went into apoptosis when they were incubated in vitro with a macrolide. A balance tipping towards apoptosis rather than to necrosis of cells in the case of acute inflammation can result in lower exposure of the immune system to cellular debris and thus limits the inflammatory response. Moreover, the increased apoptosis of PMNs may also contribute to the reduction of PMN accumulation in the lung compartment. The pro-apoptotic effect of macrolides on host cells is possibly counteracted by the release of bacterial products. However, this dual effect in macrolide action, namely priming for apoptosis of PMNs in the absence of bacterial products and the absence of apoptosis when bacterial products are present, has not been sufficiently studied to draw conclusions on its mechanism or relevance for clinical practice. The observed enhancement of the opsonophagocytic activity of PMNs and the degranulation of azurophilic and specific granules in these cells by macrolides may result in more efficient elimination of the invading microorganism in CAP and, hence, a shorter period of inflammation.
Effects of macrolides on structural cells of the respiratory tract
Studies reporting the effects of macrolides on structural cells of the respiratory tract focus mainly on the expression of adhesion molecules on the endothelium and epithelium, which aid in the migration of inflammatory cells, the integrity of the endothelium and the histological architecture of the lung.
Endothelium and epithelium
Various in vitro studies have demonstrated that macrolides affect structural lung cells at a cellular level (Table 4). In endothelium pre-treated with azithromycin and roxithromycin, and subsequently infected with Chlamydia pneumoniae or stimulated with TNF-α, significantly decreased transendothelial migration of PMNs and monocytes was found. In endothelium treated with clarithromycin this effect was absent.14 Bronchial epithelium that was treated with erythromycin after H. influenzae endotoxin challenge expressed less soluble intracellular adhesion molecules (s-ICAM), which normally would aid in PMN adherence to epithelial cells. In addition, when human endothelium was incubated with bronchial epithelium that was challenged with H. influenzae endotoxin and treated with erythromycin, a decrease in PMN adherence to endothelium was observed.19 ICAM-1 expression of bronchial cells also decreased when non-typeable H. influenzae was treated with erythromycin.15 ICAM-1 expression on human endothelial cells decreased when stimulated with supernatants from S. aureus treated with erythromycin, but not when treated with β-lactams. Erythromycin treatment also reduced PMN adhesion to endothelium, when compared with β-lactam treatment.22 Furthermore, apoptotic activity was increased in murine alveolar type II cells that were pre-treated with telithromycin and afterwards incubated with supernatants from LPS-challenged macrophages.23
Effects of macrolides on structural cells in vitro sorted by cell type
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on structural cells . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| Endothelium | human | CLR | C. pneumoniae | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | ERY | S. aureus supernatant | reduced adherence of granulocytes to endothelium | compared with β-lactams; the effect is probably the result of a change in endothelium rather than inflammatory cells | van Langevelde et al. (1999)22 |
| Endothelium | human | ERY | S. aureus supernatant | reduced expression of ICAM-1 by endothelial cells | compared with β-lactams | van Langevelde et al. (1999)22 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | decreased ICAM-1 expression | 10 mg/L ERY reduced ICAM-1 compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | decreased s-ICAM-1 expression | compared with antibiotic-free controls | Khair et al. (1995)19 |
| Alveolar type II cells | murine | TEL | LPS-stimulated macrophage supernatant | increase in apoptotic activity | compared with antibiotic-free controls | Leiva et al. (2008)23 |
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on structural cells . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| Endothelium | human | CLR | C. pneumoniae | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | ERY | S. aureus supernatant | reduced adherence of granulocytes to endothelium | compared with β-lactams; the effect is probably the result of a change in endothelium rather than inflammatory cells | van Langevelde et al. (1999)22 |
| Endothelium | human | ERY | S. aureus supernatant | reduced expression of ICAM-1 by endothelial cells | compared with β-lactams | van Langevelde et al. (1999)22 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | decreased ICAM-1 expression | 10 mg/L ERY reduced ICAM-1 compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | decreased s-ICAM-1 expression | compared with antibiotic-free controls | Khair et al. (1995)19 |
| Alveolar type II cells | murine | TEL | LPS-stimulated macrophage supernatant | increase in apoptotic activity | compared with antibiotic-free controls | Leiva et al. (2008)23 |
AZM, azithromycin; CLR, clarithromycin; ERY, erythromycin; LPS, lipopolysaccharides; PMN, polymorphonuclear leucocyte; RXM, roxithromycin; (s)-ICAM-1, (soluble) intracellular adhesion molecule; TEL, telithromycin; TEM, transendothelial migration; TNF, tumour necrosis factor.
Effects of macrolides on structural cells in vitro sorted by cell type
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on structural cells . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| Endothelium | human | CLR | C. pneumoniae | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | ERY | S. aureus supernatant | reduced adherence of granulocytes to endothelium | compared with β-lactams; the effect is probably the result of a change in endothelium rather than inflammatory cells | van Langevelde et al. (1999)22 |
| Endothelium | human | ERY | S. aureus supernatant | reduced expression of ICAM-1 by endothelial cells | compared with β-lactams | van Langevelde et al. (1999)22 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | decreased ICAM-1 expression | 10 mg/L ERY reduced ICAM-1 compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | decreased s-ICAM-1 expression | compared with antibiotic-free controls | Khair et al. (1995)19 |
| Alveolar type II cells | murine | TEL | LPS-stimulated macrophage supernatant | increase in apoptotic activity | compared with antibiotic-free controls | Leiva et al. (2008)23 |
| Cell type . | Model . | Macrolide . | Pathogen/stimulation . | Influence on structural cells . | Comments . | Reference . |
|---|---|---|---|---|---|---|
| Endothelium | human | CLR | C. pneumoniae | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | CLR | TNF-α | no effect on PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | AZM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | C. pneumoniae | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | RXM | TNF-α | significant decrease in PMN and monocyte TEM | compared with antibiotic-free controls | Uriarte et al. (2002)14 |
| Endothelium | human | ERY | S. aureus supernatant | reduced adherence of granulocytes to endothelium | compared with β-lactams; the effect is probably the result of a change in endothelium rather than inflammatory cells | van Langevelde et al. (1999)22 |
| Endothelium | human | ERY | S. aureus supernatant | reduced expression of ICAM-1 by endothelial cells | compared with β-lactams | van Langevelde et al. (1999)22 |
| Bronchial epithelium | human | ERY | H. influenzae, non-typeable | decreased ICAM-1 expression | 10 mg/L ERY reduced ICAM-1 compared with 0.1 mg/L ERY or 100 mg/L gentamicin | Qian et al. (2010)15 |
| Bronchial epithelium | human | ERY | H. influenzae endotoxin | decreased s-ICAM-1 expression | compared with antibiotic-free controls | Khair et al. (1995)19 |
| Alveolar type II cells | murine | TEL | LPS-stimulated macrophage supernatant | increase in apoptotic activity | compared with antibiotic-free controls | Leiva et al. (2008)23 |
AZM, azithromycin; CLR, clarithromycin; ERY, erythromycin; LPS, lipopolysaccharides; PMN, polymorphonuclear leucocyte; RXM, roxithromycin; (s)-ICAM-1, (soluble) intracellular adhesion molecule; TEL, telithromycin; TEM, transendothelial migration; TNF, tumour necrosis factor.
Histological architecture of the lung
Several studies evaluated the effects of macrolides on the histological signs of inflammation in murine models (Table 5). In these studies, the degree of lung inflammatory changes is classified using a uniform scoring system that assigns points to visual parameters of inflammation [lung histopathological score (HPS)] or using the individual assessment of a pathologist.
Effects of macrolides on histological signs of inflammation in murine pneumonia models sorted by pathogen/stimulation
| Pathogen/stimulation . | Macrolide . | Histopathological findings . | Comments . | Reference . |
|---|---|---|---|---|
| S. pneumoniae | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | RXM | less damage to lung tissue | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (1998)37 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | AZM | HPS significantly reduced | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | CET | HPS significantly reduced | no significant reduction of bacterial cultures in BALF | Rios et al. (2004)30 |
| M. pneumoniae, killed | CLR | no reduction in HPS | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae antigen | CLR | CLR moderated the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| M. pneumoniae antigen, macrolide resistant | CLR | CLR did not moderate the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| H. influenzae, macrolide resistant | CLR | only mild inflammatory changes were evident | compared with untreated controls | Nakamura et al. (2010)34 |
| Pathogen/stimulation . | Macrolide . | Histopathological findings . | Comments . | Reference . |
|---|---|---|---|---|
| S. pneumoniae | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | RXM | less damage to lung tissue | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (1998)37 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | AZM | HPS significantly reduced | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | CET | HPS significantly reduced | no significant reduction of bacterial cultures in BALF | Rios et al. (2004)30 |
| M. pneumoniae, killed | CLR | no reduction in HPS | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae antigen | CLR | CLR moderated the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| M. pneumoniae antigen, macrolide resistant | CLR | CLR did not moderate the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| H. influenzae, macrolide resistant | CLR | only mild inflammatory changes were evident | compared with untreated controls | Nakamura et al. (2010)34 |
AZM, azithromycin; BALF, bronchoalveolar lavage fluid; CLR, clarithromycin; CET, cethromycin; CIP, ciprofloxacin; HPS, histopathological score; MIN, minocycline; RXM, roxithromycin.
Effects of macrolides on histological signs of inflammation in murine pneumonia models sorted by pathogen/stimulation
| Pathogen/stimulation . | Macrolide . | Histopathological findings . | Comments . | Reference . |
|---|---|---|---|---|
| S. pneumoniae | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | RXM | less damage to lung tissue | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (1998)37 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | AZM | HPS significantly reduced | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | CET | HPS significantly reduced | no significant reduction of bacterial cultures in BALF | Rios et al. (2004)30 |
| M. pneumoniae, killed | CLR | no reduction in HPS | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae antigen | CLR | CLR moderated the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| M. pneumoniae antigen, macrolide resistant | CLR | CLR did not moderate the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| H. influenzae, macrolide resistant | CLR | only mild inflammatory changes were evident | compared with untreated controls | Nakamura et al. (2010)34 |
| Pathogen/stimulation . | Macrolide . | Histopathological findings . | Comments . | Reference . |
|---|---|---|---|---|
| S. pneumoniae | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (2001)32 |
| S. pneumoniae, macrolide resistant | RXM | less damage to lung tissue | compared with untreated controls | Yasuda et al. (2007)35 |
| S. pneumoniae, killed | HMR 3004 | reduced lung oedema, lung tissue resembled healthy controls | compared with untreated controls | Duong et al. (1998)37 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae | CLR | HPS significantly reduced | compared with placebo | Tagliabue et al. (2008)33 |
| M. pneumoniae | AZM | HPS significantly reduced | compared with placebo | Rios et al. (2005)29 |
| M. pneumoniae | CET | HPS significantly reduced | no significant reduction of bacterial cultures in BALF | Rios et al. (2004)30 |
| M. pneumoniae, killed | CLR | no reduction in HPS | compared with placebo | Hardy et al. (2003)31 |
| M. pneumoniae antigen | CLR | CLR moderated the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| M. pneumoniae antigen, macrolide resistant | CLR | CLR did not moderate the severity of the induced pneumonia | CIP and MIN did not decrease inflammation | Kurata et al. (2010)20 |
| H. influenzae, macrolide resistant | CLR | only mild inflammatory changes were evident | compared with untreated controls | Nakamura et al. (2010)34 |
AZM, azithromycin; BALF, bronchoalveolar lavage fluid; CLR, clarithromycin; CET, cethromycin; CIP, ciprofloxacin; HPS, histopathological score; MIN, minocycline; RXM, roxithromycin.
Conflicting results were found for infection with M. pneumoniae or its components. Clarithromycin, cethromycin and azithromycin therapy significantly reduced the lung HPS in pneumonia caused by viable M. pneumoniae.29–31,33 In another study, clarithromycin, but not minocycline or ciprofloxacin, decreased the pulmonary inflammation induced with crude antigens of M. pneumoniae.20 These results suggest that macrolides exert local immunomodulatory effects next to antibacterial effects. However, in another model, clarithromycin did not affect inflammation in pneumonia induced by a clarithromycin-resistant strain of M. pneumoniae.20 Also, treatment of pneumonia induced by non-viable M. pneumoniae with clarithromycin did not reduce the HPS.31 The proposed explanation for this is that certain bacterial strains may cause inflammation, which cannot be controlled by a macrolide.
Studies investigating the macrolide treatment of infection caused by microorganisms other than M. pneumoniae did yield consistent results. Roxithromycin treatment of murine pneumonia induced with macrolide-resistant S. pneumoniae and clarithromycin treatment of macrolide-resistant H. influenzae pneumonia resulted in less damage to lung tissue as compared with controls.34,35 Also, the ketolide HMR 3004 decreased lung oedema in a model of pneumonia caused by UV-killed S. pneumoniae to a level that was comparable to that in the uninfected control group.37 In another study, HMR 3004 administration in a model infected with viable S. pneumoniae also resulted in a decrease in pulmonary oedema formation and lung tissue from treated animals resembled specimens obtained from uninfected controls.32
Thus, macrolides affect the structural cells of the respiratory tract in the case of acute inflammation. The emerging picture is that macrolides improve endothelium integrity, which results in reduced transendothelial migration of the inflammatory cells. Furthermore, the reduced expression of adhesion molecules on both the endothelium and epithelium may lead to less effective adhesion and diapedesis of PMNs and reduced accumulation of these cells in the lung compartment. This supposed reduction in PMN accumulation in the lungs has already been confirmed in studies mentioned above. Moreover, murine studies showed that macrolides reduced the inflammatory signs caused by viable and non-viable bacteria, and even bacterial products. This not only suggests the existence of an immunomodulatory effect of macrolides in acute inflammation, but also points towards their potential use as immunomodulatory drugs in clinical practice.
Conclusions
To our best knowledge, this is the first review to discuss the immunomodulatory effects of macrolides on the immune response in CAP. Overall, the existing evidence suggests that macrolides can temper the inflammatory response independent of antibacterial activity at different levels (cytokines, inflammatory cells and structural cells). The findings from the studies discussed in this review are in favour of the use of macrolides as adjuvant therapy in CAP.
However, the available data do not allow us to draw definite conclusions about individual macrolides, microorganisms or specific cytokines, because of the heterogeneity of the studies.
Moreover, in vitro and in vivo studies evaluating the immunomodulatory effects of macrolides in combination with β-lactam therapy are lacking. Such studies would be particularly clinically relevant, since worldwide controversy exists as to whether macrolides should be added to β-lactam antibiotics empirically in the treatment of CAP. To resolve this ongoing debate, first, the exact mechanisms of macrolide immunomodulation need to be elucidated, focused on macrolides in combination with β-lactam antibiotics. Second, a randomized controlled trial is required to definitively assess the clinical impact of adjuvant macrolide therapy in CAP.
Funding
This work was performed as part of the regular professional activities of the authors and was made without specific additional funding.
Transparency declarations
None to declare.